Welcome to an exciting round of “name that component”. Here’s your host, Tito!
Hi everybody,
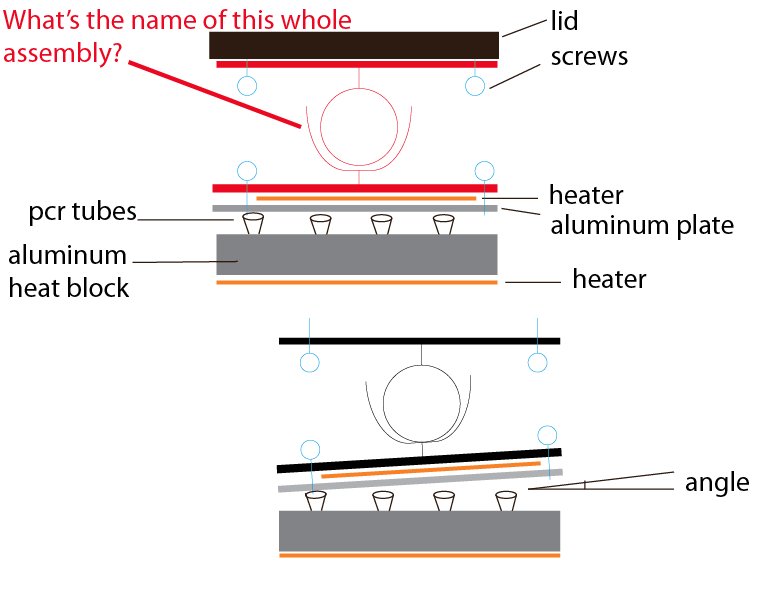
We are working to design our heated lid so that it can adjust to small differences in the size or height of tubes. In PCR, we’re cycling the temperature of a small aluminum block between 50C and 100C. We place a plastic tube in each well of the aluminum block, which contains a liquid DNA sample. Now, if we simply have this setup, we will heat our samples to 100C and they will evaporate and condense in the lid of the tube. This is a problem, which is commonly solved by something called a “heated lid”. The 100 C lid needs to make contact with the caps of the PCR tubes, to prevent condensation of the sample. At this point, we have a flat aluminum plate with a heater mounted to it, and are able to reach 100 C. The issue is having the plate make flat contact with all 16 (4 x 4) tubes in the OpenPCR. I’m looking for some sort of a ball joint that we can mount the plate to so that it will rotate every so slightly (10 degrees would be enough).
Now, I know I’ve seen a part like this in existence, but we have little idea where to find it or what is it called?
I would describe it simple as 2 flat plates with a ball joint in between. The size of the plates should be smaller than 1.5″ square, with a few holes in each plate for mounting. I’ve drawn up a crude illustration to attempt at describing this, and have searched mcmastercarr (“ball socket”) got me the closest, but no cigar.
Here’s some “similar” products to what I have in mind…but looking in the <$10 price range and much smaller: https://www.thorlabs.com/thorProduct.cfm?partNumber=SL20
(car gps mount) https://www.cabelas.com/p-0012344012724a.shtml
https://www.newport.com/RN-Series-Ball-and-Socket-Stages/144558/1033/catalog.aspx
Hope you have ideas in mind!
Tito
I understand your need to achieve even pressure/contact on top of your tubes even with possible misalignment of the lid, although I’m not sure the best solution is some ball and socket type arrangement.
I assume the root of your problem is that these tubes were not designed to have heat transfered to them thru their top caps and as a result height, flatness, angle etc of the caps varies wildly. So long as your heater/plate assembly is flat then no matter how it is articulated compared to the lid, only the highest three tubes will be in thermal contact with the heater/plate. I presume this is why most PCR thermal cyclers have some metallic egg-crate looking piece which holds the tubes and has a flat base which is in contact with the peltier devices.
If you are set on using the top as the heat source – maybe something like a bed of retracting pins that touches each tube might guarantee contact. Or maybe something compliant but thermally conductive – many small copper tabs which act like leaf springs and push on the top of each tube? If a conductive pillow existed…
Or you could try adapting a ball-socket joint although I couldn’t quickly find anything too general. Basically lots of things used as camera tripods, electronics vises etc. You could use standard ball-joint (sometimes called rod end) parts but all this just seems kind of Rube-Goldberg. I linked a few McMaster options but most large bearing manufacturers make a range of ball joints intended as rod ends for non-planar mechanical linkages.
https://www.mcmaster.com/#rod-ends/=8offlg
click “ball-joint linkages” -> “inline ball joint linkages”
https://www.mcmaster.com/#ball-sockets/=8of1kj
https://www.atvresearch.com/cwmtdoubleball-jointindoorcameramount.aspx
https://www.panavise.com/index.html?pageID=1&page=full&–eqskudatarq=2
Maybe mount the plate to the lid with helical springs to let the plate naturally adjust angle when it comes down on the tubes? If that was too sloppy then you could add a few links which allow lots of relative motion between the lid and plate but ultimately constrain the range of motion.
Sorry this probably not exactly the most helpful response
Sorry, another point about why it might be good to heat the sockets which hold the tubes. In that arrangement hear is transferred thru the plastic tube to the liquid via conduction. If the liquid in the tube is not in contact with the cap for the tube than all heat must be transfered down the tube itself before contacting the liquid, rather than merely traveling thru the thickness of the tube. I don’t know how much of a difference this makes practically but it intuitively seems slower – possibly significantly slower and more difficult to control.
Hi Kyle,
Thanks for your comments!
First, let me clarify. The tubes *are* heated from below by the solid aluminum block. The heated lid at the top is to prevent condensation in the tubes. As the aluminum block on the bottom heats up, the sample would condense at the top of the tube, away from the heat source, if it weren’t for the heated lid. I think we’re on the same page, what you described is in fact what our design is (works much like most PCR machines).
(This sketch might help explain: https://picasaweb.google.com/TitoJankowski/KickstarterUpdate#5503164607746352594
I took a look at the rod ends and ball sockets, thanks for the suggestions. Like you said, the problem is that rod ends are designed for something entirely different, and we would have to design a contraption to make use of the parts. The camera mount is a lot warmer, maybe something smaller with a bit different design?
Tito
Hi Tito,
First I think you’ll find that most PCR tubes, of all sizes, are machined quite uniformly as the requirements are pretty tight. The biggest problem I have with tubes is merely finding tubes that have the resistance to the ABI 9700 lid which reaches 104-105 and will warp most .2mL tubes.
But, moving into your problem, I think that your approach is interest but possibly too complex/rigid for what you may want. You are trying to fix a problem where one tube on one side might be slightly shorter/taller than the rest needing a tilted lid. Ignoring the mentioned QC of tubes, what if all the tubes are shorter than your lid design? or taller? will you get a proper lock on the lid at all with a ball joint?
(Here’s where my background in molecular biology ceases to aid my vocabulary)
I think a possible, and cheaper, solution would be to use four small spring mounts. This would give your lid both tilt and height variability, also creating a slight pressure against the lids assisting with seal.
That’s a good suggestion, David. I’ll see if I can find any small spring mounts. Let me know if you have a particular vendor in mind, the ones I’ve seen so far are really large.
On the other hand, it might be just as easy to put 4 bolts around the edges of the lid, and put little springs in there by hand.
Tito
I have a few ideas, although I don’t know very much about the mechanics inside the machine. What I was thinking was to put a grid of small springs under the test tubes so that there could be variable pressure for different (minor) variations in tube height. This would ensure some pressure of the tubes on the lid.
The other idea is to do away with the ball joint and instead have four short stiff springs arranged in a square, either in an X or a + configuration between the lid layers. This should provide consistent-enough pressure while not making the coupling between inner and outer lid rigid.
The third idea, and this material may not actually exist, is to line the lid with the same or similar material, a woven metal mesh that is used to ensure electrical contact between different computer components and the chassis or case. This material should be thermally conductive enough to be useful while also decreasing condensation formation by providing spaces between the mesh fibers to let the vapor escape (unless that is something you don’t want to happen). The lid could still be sealed with a gasket, but again, I’m not completely familiar with the entire design.
for different height tubes (assuming all the tubes in a run are the same height) why not just use a simple linkage?
https://fennetic.net/irc/pcr_heated_lid.png
if you’re dead set on the ball joint design, you could just make two plats with a hole and put a ball bearing between them.
another idea is to use some nice rubber bands around tabs sticking out of corners of the lid. I wouldn’t want to have to undo a bolt with a spring every time I opened the lid. though I guess you could do some kind of swiveling latch out of laser cut pieces.
for the thermally conductive yet squishy material, try high temperature silicone foam rubber with adhesive backing, i.e. mcmaster part number 5109K36
Pingback: The heated lid | OpenPCR
Try https://www.leespring.com for off the shelf spring selection.
Just to ask can you tell where your dna comes from ?As far countries are concerned.
sincerely Tony
Hi guys, first all congratulations for your awesome work.
The problem I see is: you need a heat lid that adjust a different kinds of PCR tubes (0.2 or 0,6 ml), but not at same time, because none uses different PCR tubes in the same experiment. I think you can make a design with coils or springs (I’m not sure of correct name in english). There are a kind of wave springs that you can use but not sure about the price. This is a model for the heat lid (https://www.cycwebsolutions.net/images/heat_lid.jpg). The place were you can find it (or others ones) is https://www.leespring.com/uk_int_learn_wave.asp.
I Apologize for my english.
Good luck!
we deals in Electronics Products in India, Having Dealer Network in almost every state of India.
we are interested , Open PCR,
kindly send Rates of the same, along with other terms and Condition of business.
This is an easy fix. You can just place 10-20 uL of Mineral Oil in the tube of your sample before running your PCR. It is non reactive so it won’t affect your sample. The oil just stays on top of your sample and prevents your sample from evaporating. We do this in our lab all the time when the heating lid of our $10,000 PCR machine breaks.